Multiple myeloma (MM) is a genetically heterogeneous disease characterized by plasma cell clonal proliferation. Due to the potential complex cytogenetic abnormalities, the clinical progresses are heterogeneous. Thus, genome-wide identification of cytogenetic abnormalities is necessary given the limitations of traditional cytogenetic detecting methods. In this study, we utilized whole genomic mapping methods Cytoscan 750K array to analyze the genomic copy number variations (CNVs) of 611 Newly Diagnosed MM (NDMM) from The First Affiliated Hospital of Soochow University.
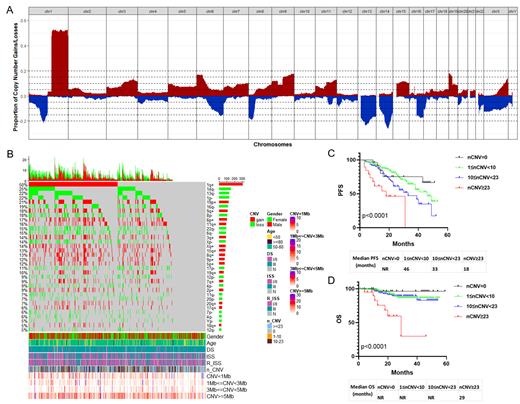
The results showed that 95.3% of NDMM patients harbored the chromosome alterations including structural or numerical abnormalities, and most of them (67.3%) were accompanied both, including 23.7% with only structural abnormalities and 4.3% with only numerical abnormalities. The average number of CNV per patient was 9. In addition, we have identified 34 common CNV events with a frequency of ≥5%, 5 of which were high frequency (≥20%), including dup(1)(q21q44) (50.2%), del(14)(q22q32) (24.9%), del(1)(p31p12) (21.9%), del(13)(q12q22) (21.9%) and dup(19)(p13p12) (20.6%). Additionally, 6 CNVs with median frequency (≥15% and <20%) included dup(6)(p25p12) (16.9%), dup(9)(q21q34) (18.2%), del(16)(q12q24) (18.7%), del(8)(p23p12) (17.5%), dup(11)(q13q25) (15.7%) and del(22)(q11q12) (15.4%). And the other 23 CNVs were low frequency (<15%), including del(6)(q24q27) (14.6%), del(X)(p22q25) (14.1%), dup(X)(q28) (13.7%), dup(8) (q11q24) (12.6%), del(17)(p13p12) (11%) and so on.
Most CNVs were closely related with clinical risk factors of MM such as LDH. Prognostic analysis based on the VRD treatment (n=353, the median follow-up was 22 months) showed that patients with only numerical abnormalities had a better progress free survival (PFS) outcome than that with structural abnormalities only or with both, although they seemed to have the similar overall survival (OS) rate. Furthermore, univariate analysis showed that 6 CNVs were correlated with poor PFS including 1q+, 4q-, 6q-, 12p-, 16q-, 17p-, and 5 CNVs were correlated with poor OS including 1p-, 4q-, 7p-, 12p-, 17p-.
For the effect of counts of CNV events on MM prognosis, the receiver operating characteristic (ROC) curve analysis was performed to calculate the best cutoff, with a result of 23 CNV events. Therefore, MM patients were divided into 4 subgroups according to the counts of CNV they harbored, including high complexity group (CNVs≥23, n=32) which excluded patients with chromothripsis (more than 10 CNVs on the same chromosome) , median complexity group (10≤CNVs<23, n=168), low complexity group (1≤CNVs<10, n=324), and no CNVs group (n=73). The patients in high complexity group had the worst prognosis of PFS and OS (media PFS 18 months, media OS 29 months, P<0.001). As expected, some common high risk clinical factors such as 1q+, 17p-, DS III, ISS III, serum Ca++,β2-MG and plasma cells in BM were gradually increased among the group from low to high complexity, but decreased in the rate of complete remission (CR), which suggested that the risk classification system was reliable. Importantly, univariate and multivariate COX regression analysis showed that CNV≥23 was an independent prognostic risk factor affecting both PFS and OS in NDMM patients.
Over all, we utilized a large MM cohort to identify recurrent and potential driver CNV events, and their correlation with prognosis. In addition, the count of CNVs in NDMM patient was creatively considered to be the basis for prognostic risk stratification.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal